Autonomous artificial intelligence increases screening and follow-up for diabetic retinopathy in youth: the ACCESS randomized control trial
Abstract
Diabetic retinopathy can be prevented with screening and early detection. We hypothesized that autonomous artificial intelligence (AI) diabetic eye exams at the point-of-care would increase diabetic eye exam completion rates in a racially and ethnically diverse youth population. AI for Children’s diabetic Eye Exams (NCT05131451) is a parallel randomized controlled trial that randomized youth (ages 8-21 years) with type 1 and type 2 diabetes to intervention (autonomous artificial intelligence diabetic eye exam at the point of care), or control (scripted eye care provider referral and education) in an academic pediatric diabetes center. The primary outcome was diabetic eye exam completion rate within 6 months. The secondary outcome was the proportion of participants who completed follow-through with an eye care provider if deemed appropriate. Diabetic eye exam completion rate was significantly higher (100%, 95%CI: 95.5%, 100%) in the intervention group (n = 81) than the control group (n = 83) (22%, 95%CI: 14.2%, 32.4%)(p < 0.001). In the intervention arm, 25/81 participants had an abnormal result, of whom 64% (16/25) completed follow-through with an eye care provider, compared to 22% in the control arm (p < 0.001). Autonomous AI increases diabetic eye exam completion rates in youth with diabetes.
Diabetic retinopathy is a complication of diabetes that can be prevented through screening, yet adherence is low. Here, the authors show that autonomous AI increases diabetic eye exam completion in a diverse cohort of youth with diabetes.
Introduction
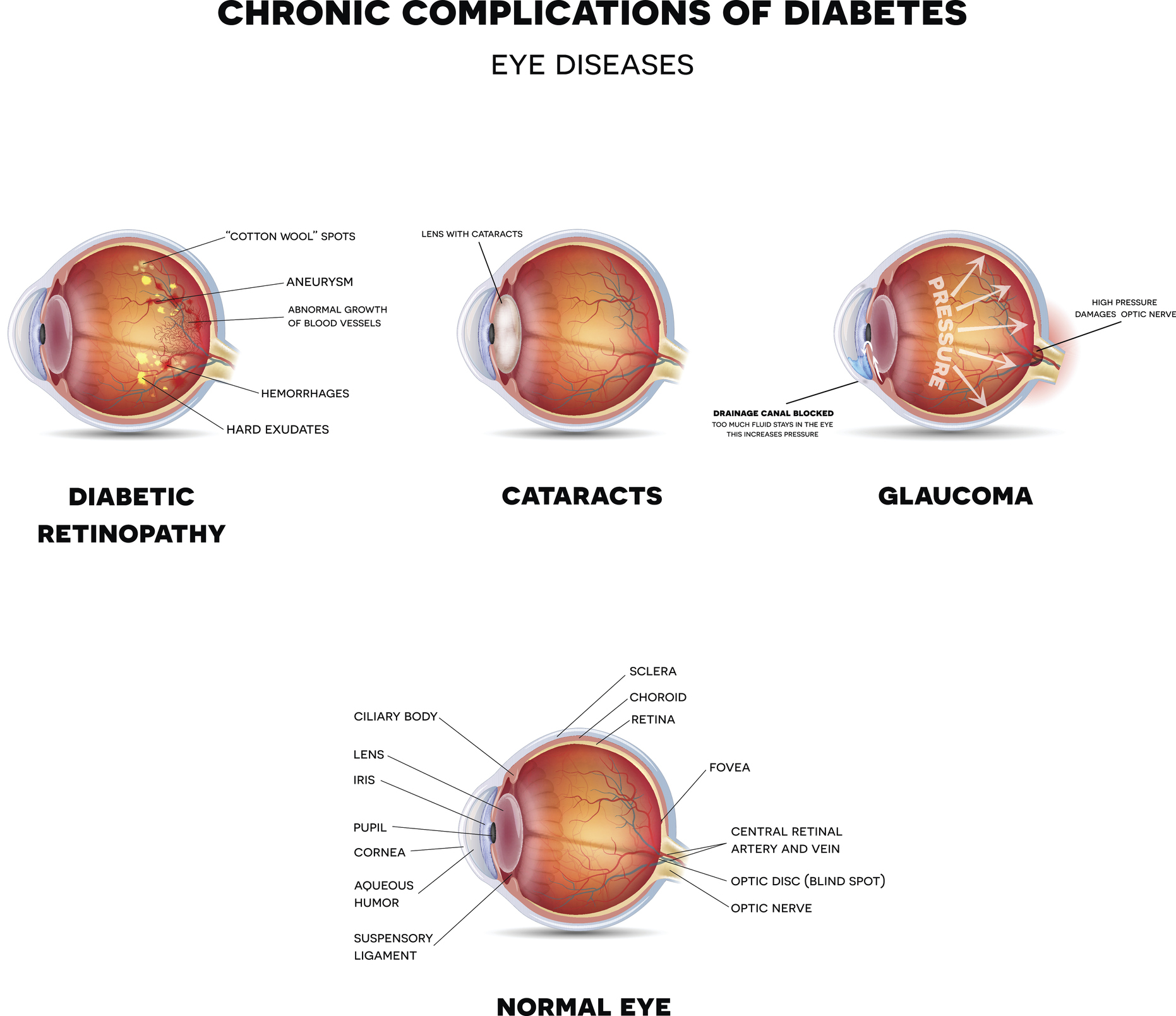
Diabetic eye disease (DED) is a complication of diabetes that is the primary cause of blindness in working-age adults in the U.S.1,2. Early detection (‘screening’) and treatment can frequently prevent progression, but the majority of the 34 million people with diabetes in the US have a DED screening care gap, due to a lack of access, and education around the need for a diabetic eye exam3,4. This care gap is a major source of health disparity, with racial and ethnic minorities, and under-resourced communities, having worse outcomes, and a disproportionately higher prevalence of DED2,5–9.
While DED prevalence is lower in youth (defined as those aged <21 years) with diabetes, it affects approximately 4–9% of youth with type 1 diabetes (T1D) and 4-15% of youth with type 2 diabetes (T2D)10–14. The risk for DED increases with the duration of diabetes in T1D, and recent data from the TODAY2 follow-up study demonstrated a diabetic retinopathy prevalence rate of 49% at a mean diabetes duration of 12 years in youth onset T2D15. The American Diabetes Association (ADA) and American Academy of Ophthalmology (AAO) recommend DED screening in T1D within 3–5 years of diagnosis and age greater than 11 years, and in T2D at the time of diagnosis in youth16. However, only 35-72% of diabetic youth undergo recommended screening exams, with even higher care gap rates in minority and lower socioeconomic background youth10,17. Commonly reported barriers to screening include miscommunication regarding the need for a diabetic eye exam, time for an additional doctor’s visit, and transportation barriers17,18.
While the introduction of telemedicine over the last two decades has improved screening and facilitated early detection of diabetic eye disease19–23, the development of diagnostic autonomous artificial intelligence (AI) systems for diagnosing DED has ushered in the next chapter of DED screening24–29. Autonomous AI systems require a camera operator to obtain point-of-care fundus images, which are then interpreted by an AI algorithm to provide a diagnosis without human oversight. The regulatory approval of the first diagnostic autonomous AI system for diabetic eye exams was based on a pivotal trial against a prognostic standard, i.e., patient outcome24, showing its safety, efficacy, and lack of racial and ethnic bias for diagnosing DED in adults with diabetes with 87% sensitivity and 91% specificity24. In prior studies of youth with diabetes, we demonstrated that the diagnosability of autonomous AI in youth was 97.5%, with 85.7% sensitivity and 79.3% specificity in detecting DED, with no difference in diagnosability across demographic groups30. We have also shown that this system can be implemented in a multidisciplinary diabetes clinic and has the potential to increase DED screening rates in underserved youth, while also being cost-savings to patients and caregivers30,31.
While diagnostic accuracy has been a focus of study of diagnostic AI systems25,30,32, the effectiveness of autonomous AI to increase adherence and follow-up compared to traditional referral has not been evaluated in a rigorously designed randomized trial. We hypothesized that autonomous AI closes the diabetic eye exam care gap, and increases follow-up, compared to traditional eye care provider(ECP) referral in youth. To test this hypothesis, we designed ACCESS (AI for Childrens’ diabetiC Eye examS Study), a pre-registered, rigorously designed randomized control trial (RCT), to measure diabetic eye exam completion rates in a racially and ethnically diverse cohort of youth with T1D and T2D.
news via inbox
Subscribe here to get latest updates !